
02-01-2008, 12:05 AM
|
 |
مدرس الكيمياء للثانوية الازهرية
|
|
تاريخ التسجيل: Jan 2007
المشاركات: 1,960
معدل تقييم المستوى: 0
|
|
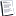
Types of chemical bonds
* IONIC BOND
- It is usually formed between metals and non- metals.
- Metals
The atom is large in volume- but small in ionization energies and electronegativity so it loses electrons from outer level converting into positive ion (cation).
Non - metals
The atom is small in volume - but high ionization energy and electronegativity. So it gains electrons converting into negative ion (anion).
* Ionic bond
An electrostatic attraction between positive ion (cation) and negative ion to form neutral compound.
N.B.
- As the difference in electronegativity increases between the binding elements (horizontally), the ionic character increases
In ionic compounds the difference in electronegativity >1.7
* General properties of ionic compounds:
I- Structure
Crystal lattice containing the ions in regular pattern
II- Melting and boiling points
They have high melting and boiling points because a great energy is required to break up the attraction between ions
III- Solution in polar solvents
Crystal lattice is broken down (dissolve) in polar solvents
IV- Electrical conductivity
They conduct electricity through free ions in molten or hydrated ions in aqueous solutions.
__________________
 دكتور عاطف خليفة
كيميائي
500 امتحان كيمياء
دكتور عاطف خليفة
كيميائي
500 امتحان كيمياء
|