
29-09-2008, 08:13 PM
|
 |
مدرس الكيمياء للثانوية الازهرية
|
|
تاريخ التسجيل: Jan 2007
المشاركات: 1,960
معدل تقييم المستوى: 0
|
|
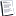
Reversible Process
A reversible process is one in which the timescale is assumed to be so slow that every intermediate state deviates only infinitesimally from equilibrium. Every intermediate state is exactly described by a set of macroscopic thermodynamic variables and may be assumed to be at equilibrium. Since every intermediate state is exactly known, the process may be reversed at an infinitesimally slow rate. This may be simply illustrated by imagining a cylinder with a frictionless piston on the top. Further imagine that there is a quantity of sand on top of the piston. A good approximation to a reversible process would be realized by removing the sand one grain at a time and carefully recording the thermodynamic variables (temperature and pressure in this case) after each grain of sand is removed. This would be a reversible expansion and one could individually return the grains of sand one at a time and reproduce each intermediate state exactly, thus reversing the transformation.
Equilibrium
Equilibrium Constant
The equilibrium constant for a chemical reaction is given by
where  is the Helmholtz free energy, k is the Stefan-Boltzmann constant, and T is the temperature is the Helmholtz free energy, k is the Stefan-Boltzmann constant, and T is the temperature
Helmholtz Free Energy
The Helmholtz free energy is defined by  (1)
where E is the energy, T is the temperature, and S is the entropy. When a system changes its thermodynamic state, the change in Helmholtz free energy is therefore given by  (2)
If T and V are constant, the (2) reduces to  (3)
But the combined law of thermodynamics states that  (4)
and, since we have stipulated dV = 0, this becomes  (5)
Therefore  (6)
The Helmholtz free energy is intimately related to the equilibrium constant at constant volume  via via  (7)
where k is Boltzmann's constant. A system with fixed external parameters in thermal contact with a heat reservoir at equilibrium has a minimum Helmholtz free  energy, commonly denoted energy, commonly denoted
__________________
 دكتور عاطف خليفة
كيميائي
500 امتحان كيمياء
دكتور عاطف خليفة
كيميائي
500 امتحان كيمياء
|