
29-09-2008, 03:42 AM
|
 |
مدرس الكيمياء للثانوية الازهرية
|
|
تاريخ التسجيل: Jan 2007
المشاركات: 1,960
معدل تقييم المستوى: 0
|
|
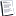
Time-Temperature Calculation #4:
Liquid Water Boiling to Gas
72.0 grams of liquid water is at 100.0 °C. It is going to boil AND stay at 100 degrees. This is an important point. While the water boils, its temperature will remain the same. We need to calculate the energy needed to do this.
This summarizes the information needed: DHvap = 40.7 kJ/mol
The mass = 72.0 g
The molar mass of H2O = 18.0 gram/mol
The calculation needed, using words & symbols is:
q = (moles of water) (DHvap)
We can rewrite the moles of water portion and make the equation like this:
q = (grams water / molar mass of water) (DHvap)
Why is this equation the way it is? Think about one mole of liquid water. That amount of water (one mole or 18.0 grams) needs 40.7 kilojoules of energy to boil. Each mole of liquid water needs 40.7 kilojoules to boil. So the (grams water / molar mass of water) in the above equation calculates the amount of moles. I hope that helped.
With the numbers in place, we have:
q = (72.0 g / 18.0 g mol¯1) (40.7 kJ / mol)
So we calculate and get 162.8 kJ. We won't bother to round off right now since there is one more calculation to go. We're doing the fourth step now. When all five are done, we'll sum them all up.
One warning before going on: three of the calculations will yield J as the unit on the answer and two will give kJ. When you add the five values together, you MUST have them all be the same unit. In the context of this problem, kJ is the preferred unit.
__________________
 دكتور عاطف خليفة
كيميائي
500 امتحان كيمياء
دكتور عاطف خليفة
كيميائي
500 امتحان كيمياء
|